Exosome-Based Therapeutic Enters First Clinical Trial in Korea... Spotlight on Industry-Academia Joint Research Achievement
- 글번호
- 406233
- 작성일
- 2025-05-15
- 수정일
- 2025-05-15
- 작성자
- 홍보팀 (032-835-9490)
- 조회수
- 346
The basic research collaboration between Professor Jaemin Cha of the Department of Bio-Robotics and Systems Engineering at Incheon National University and SNE Bio Co., Ltd., a company specializing in exosome-based therapeutics, has garnered significant attention as it led to the first-ever clinical trial approval for an exosome therapy in Korea.
Recent studies have shown that exosomes secreted by stem cells are one of the key mechanisms behind the therapeutic effects of stem cell treatments. Compared to the transplantation of live stem cells, exosome-based therapies are not only safer but also demonstrate equal or superior efficacy. Thanks to these advantages, exosomes are gaining global attention as a next-generation therapeutic platform and a potential alternative to traditional stem cell therapies. As a result, preclinical research on exosome-based treatments is actively underway worldwide.
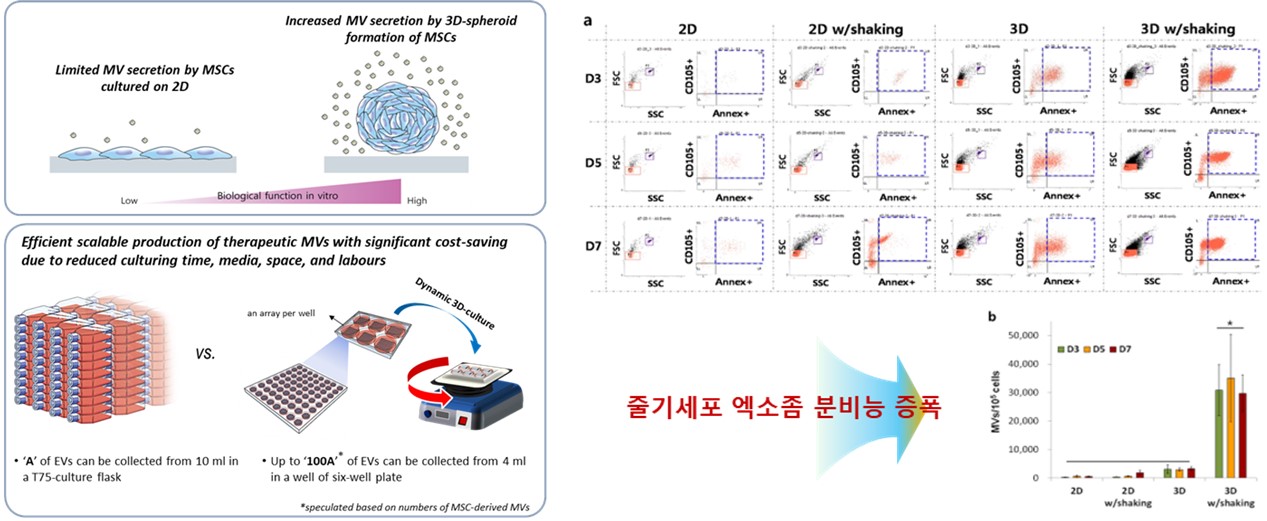
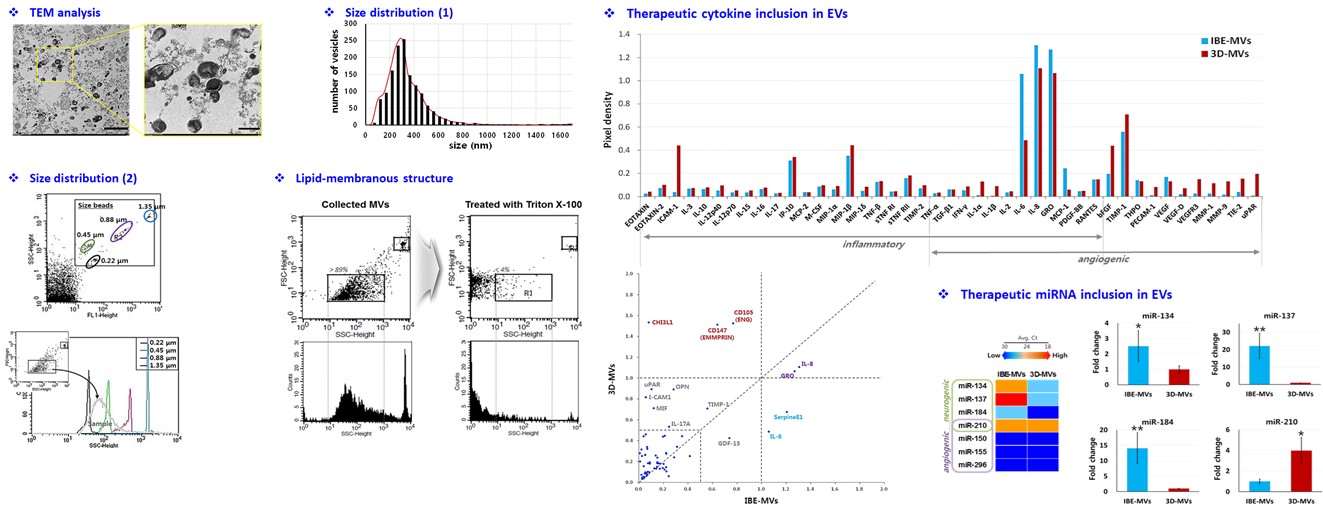
However, exosomes produced from conventional two-dimensional cultures of stem cells tend to yield low production volumes and relatively low levels of active substances, highlighting the need for improved culturing techniques. Through joint research and development, SNE Bio and Professor Cha confirmed that three-dimensional cultures not only increase the concentration of active components in exosomes but also improve production efficiency. Based on these findings, SNE Bio successfully developed a GMP-compliant mass production process for clinical application.
With support from the Ministry of Trade, Industry and Energy and the Regenerative Medicine National Consortium, SNE Bio established a GMP manufacturing process that satisfies the critical quality attributes (CQAs) for SNE-101 as required by regulatory authorities. The company ensured batch-to-batch consistency and demonstrated both efficacy and safety. As a result, on April 15, the Ministry of Food and Drug Safety approved the clinical trial protocol for SNE-101, an exosome-based treatment for acute ischemic stroke, marking the first clinical entry of an exosome therapy in Korea.
Professor Jaemin Cha of the Department of Bio-Robotics and Systems Engineering at Incheon National University has been conducting research since 2005—nearly 20 years—on three-dimensional culture technologies that simulate the in vivo environment to regulate the behavior of various stem cells, ranging from embryonic stem cells to adult stem cells. In this field, he has published over 50 SCI(E) papers in top-tier journals listed in the JCR, including ACS Nano, Advanced Materials, Advanced Healthcare Materials, Biofabrication, Translational Stroke Research, and Biomaterials Research, and holds numerous domestic and international patents.
Professor Cha has been involved with the founding of SNE Bio Co., Ltd. and has continued to collaborate with the company on exosome material and process development through university-industry joint research initiatives.